Alarcón, B., de Armas, R., Vicente, C., Legaz, M.E.
Inhibition by Substrates of a Coniferyl Alcohol Dehydrogenase Purified from Sugarcane Stalks
Current Enzyme Inhibition, 15, 1-9.
DOI: -
RESUMEN
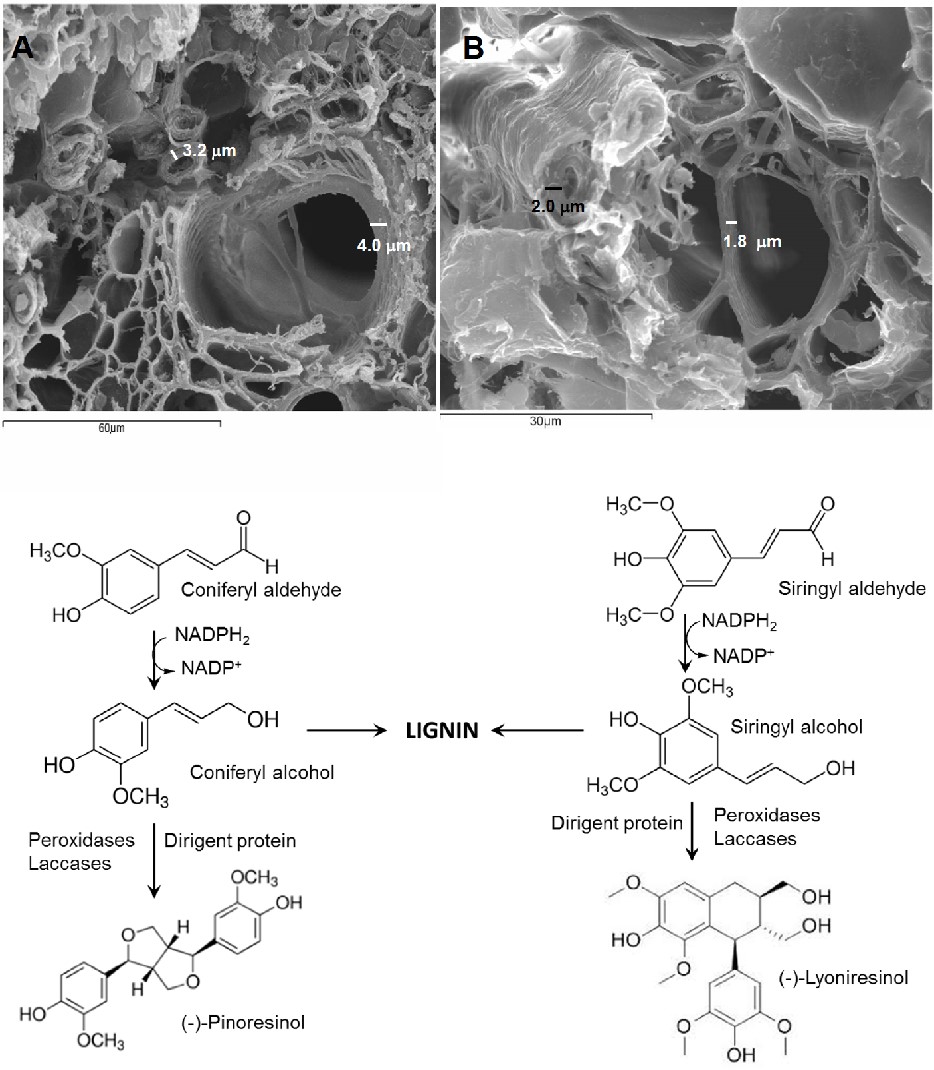
Aims & Objectives: This study aimed to characterize a coniferyl alcohol dehydrogenase from sugarcane stalks. Also, the purification of CAD from sugarcane stalks was also carried out to study kinetic properties and substrate specificity. Background: Sugarcane plants contain an alcohol dehydrogenase able to reduce both coniferyl and sinapyl aldehydes to their correspondent alcohols, although there are reasonable grounds for suspecting that these are two distinct enzymes. Methods: The enzyme, coniferyl alcohol dehydrogenase was 125-fold purified from sugarcane stalks. Its activity was estimated by HPLC by calculating the amount of product formed. Results: The enzyme showed an optimum pH value of 7.9, at an optimum temperature of 20-22 °C and a molecular mass of 48 kDa. The Km value for coniferyl alcohol was 3.03 µM and the enzyme was shown to be inhibited by an excess of the substrate from 17 µM. This dehydrogenase showed a similar affinity to sinapyl alcohol (Km 1.78 µM). Conclusion: This paper provides circumstantial evidence about the existence of two different alcohol dehydrogenases, specific to each of the substrates.