Gatta AT, Olmos Y, Stoten CL, Chen Q, Rosenthal PB, Carlton JG.
CDK1 controls CHMP7-dependent nuclear envelope reformation.
Elife. 2021 Jul 21;10:e59999
DOI: 10.7554/eLife.59999
RESUMEN
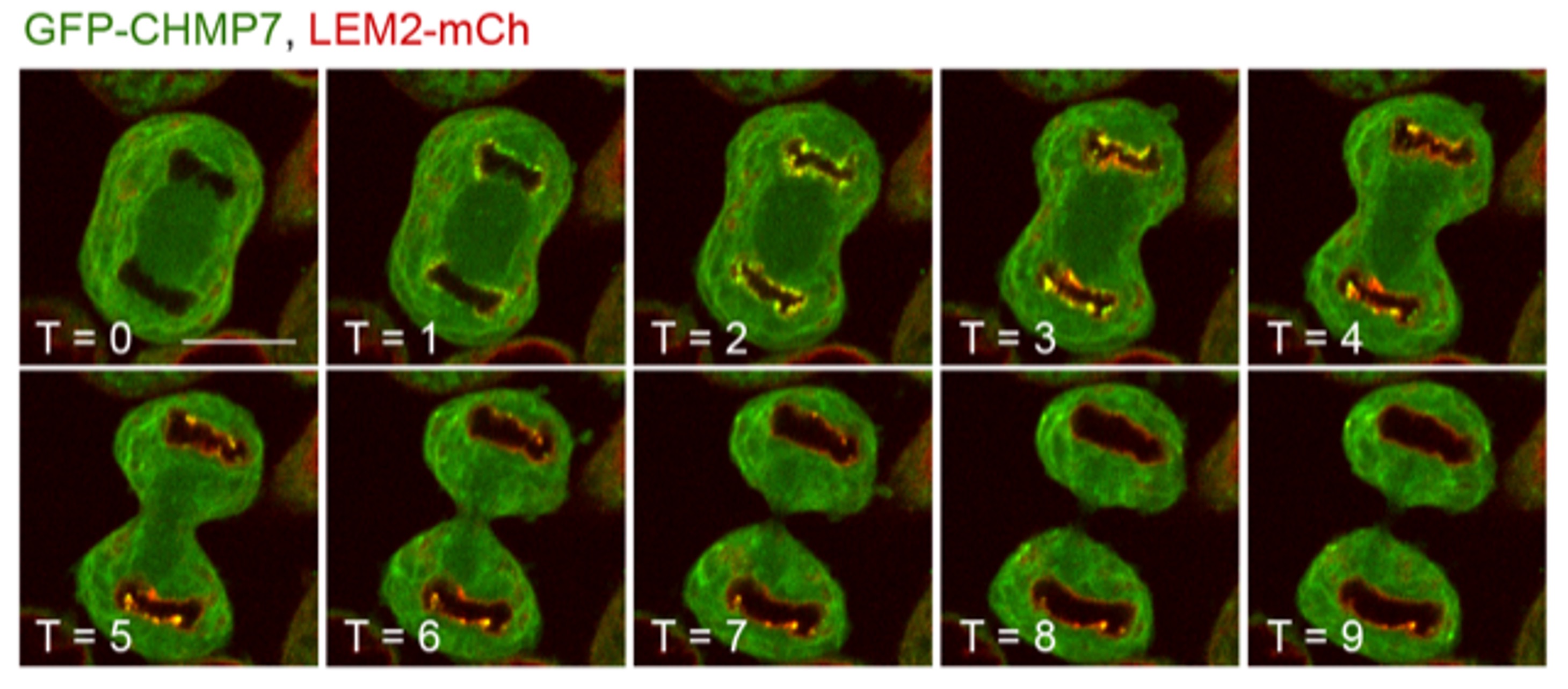
The ESCRT machinery is a key player in the regeneration of the nuclear envelope during mitotic exit, and in the repair of this organelle during interphase rupture. ESCRT-III assembly at the NE is initiated when CHMP7, an ER-localised ESCRT-II/ESCRT-III hybrid protein, interacts with the Inner Nuclear Membrane protein LEM2. Whilst classical nucleocytoplasmic transport mechanisms have been proposed to separate LEM2 and CHMP7 during interphase, it is unclear how CHMP7 assembly is suppressed in mitosis when NE and ER identities are mixed. Here, we use live cell imaging and protein biochemistry to examine the biology of these proteins during mitotic exit. Firstly, we show that CHMP7 plays an important role in the dissolution of LEM2 clusters that form at the NE during M-exit. Secondly, we show that CDK1 phosphorylates CHMP7 upon mitotic entry at Ser3 and Ser441 and that this phosphorylation reduces CHMP7’s interaction with LEM2, limiting its assembly during M-phase. We show that spatiotemporal differences in the dephosphorylation of CHMP7 license its assembly at the NE during telophase, but restrict its assembly on the ER at this time. Without CDK1 phosphorylation, CHMP7 undergoes inappropriate assembly in the peripheral ER during M-exit, capturing LEM2 and downstream ESCRT-III components. Lastly, we establish that a microtubule network is dispensable for ESCRT-III assembly at the reforming nuclear envelope. These data identify a key cell-cycle control programme allowing ESCRT-III-dependent nuclear regeneration.